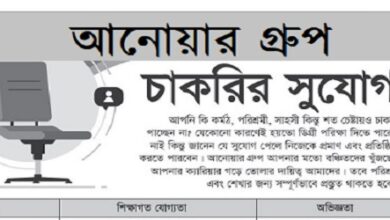
Silva Pharmaceuticals Limited Job Circular
Silva Pharmaceuticals Limited Job Circular 2021 has published from its official website and bd jobs portal website. Their authority new vacancy notice. career opportunity. admit card download. exam result at https://jobsholders.com. Non-government bank jobs are very attractive jobs here for all unemployed people. Silva Pharmaceuticals Limited jobs convert to an image file.
You know https://jobsholders.com published all jobs circular. Silva Pharmaceuticals Limited job circular publish now. Those Who wants to join this requirement can apply on this circular. We publish all information of this job. We publish Silva Pharmaceuticals Limited Job circular on our website. And get more gov and non-govt job circular in Bangladesh on our website.
Post Name: Deputy/ Quality Assurance Manager
Published on: 10 Jan, 2022
Application Deadline: 9 Feb 2022
Vacancy: N/A
Description
- To manage, co-ordinate and direct all the activities of Quality Assurance department
- To lead the Quality Assurance team to assure Quality of Products manufactured at plant.
- To lead and manage all aspects of Operational Quality, Validation, Regulatory and Technical Support and Quality Assurance services.
- Promote Quality compliance and Quality Management System (QMS).
- Take overall responsibility of Product release to market Quality Compliance, Quality Council, Technology Transfer.
- Take responsibility for Audit & Inspection of manufacturing & supply units and to attend regulatory audits & Inspections by Internal and external bodies and lead the compliance.
- Lead a team of Qualified Persons (QP) ensuring training & development of quality professionals ensuring a robust talent development and succession plan.
- Ensure that Quality Risks are proactively managed.
- Create a healthy & safe environment to work and ensure that all the EHS standards are understood and applied.
- Maintain liaison with the government & non government’s agencies.
- Lead New Technology Transfer products to be introduced to fulfill commercial ambitions.
- Handle QMS documents like product complaint, deviation, change control, reprocess, repacking etc.
- Manage proper distribution and implementation of updated SOPs and other documents.
- Implement the retention sample management system.
- Review the batch documents for batch release.
- Independently manages review and approval of master batch records, labels, specifications, and other manufacturing documents of clinical and commercial drug substances and drug products in compliance with all applicable requirements.
- Independently manages review and approval of executed manufacturing records, analytical data, and associated documentation, including deviations and investigation reports related to production and disposition.
- Leads, trains others and approves Quality investigations.
- Leads the review and approval of change control requests to ensure compliance with all applicable requirements.
- Independently initiates and approves SOP updates and creation of new SOPs. Additionally, oversees review of SOPs and associated documentation to ensure biennial review requirements are met.
- Lead establishment of Quality-specific documents (e.g., Quality Risk Management Plan, Quality Agreements, Site Master File, and Validation Master Plan).
- Ensure that all quality and regulatory requirements (as per cGMPs, ICH and ISO 9001 standard) is effective, implemented and continuously improving for all products and processes.
- Develop and maintain internal audit program and standing systems, which deal with nonconforming product (NCR), customer inquires (CI), corrective and preventive actions (CAPA), track and monitor these actions until closure.
- Schedule and conduct Management Review Meeting as required by ISO 9001 standard.
- Additional duties as assigned.

Educational Qualification:
- Master of Pharmacy (M.Pharm)
- M.Pharm from renowned University having the A-Grade Registration of Bangladesh Pharmacy Council (BPC)
- Skills Required: CAPA, cGMP, Compliance, GMP, good communication skill, MS Office, QUALITY ASSURANCE
Experience Requirements
- At least 8 year(s)
- The applicants should have experience in the following area(s):
CAPA, cGMP, good communication skill, MS Office, Pharmaceuticals, QMS, QUALITY ASSURANCE, Quality compliance, Quality Management - The applicants should have experience in the following business area(s):
Pharmaceuticals
Additional Requirements
- Age at least 40 years
- 8 to 12 years practical work experience in any reputed Pharmaceuticals in relevant field
- Excellent working knowledge of global GMP regulations, ICH guidelines and robust Quality Systems.
- Broad experience in the pharmaceutical industry with a strong analytical and / or manufacturing background and well conversant in MS Office.
- Good interpersonal and communication skills.
- Candidate must possess excellent planning & execution skills as well as strong quantitative & qualitative analytical skills.
- Strong personal & professional ethical values are essential for the position.
- Capable to work in a team and in shifting environment.
Job Location
Noakhali
Salary
- Negotiable
Compensation & Other Benefits
- Mobile bill, Tour allowance, Medical allowance, Provident fund, Gratuity
- Lunch Facilities: Full Subsidize
- Salary Review: Yearly
- Festival Bonus: 2
- Career Growth Opportunity, Dormitory, Cell Phone Allowance, Lunch Facilities, Festival Bonus, Provident Fund, Insurance, Gratuity & other admissible benefits as per company policy
Jobs Source: online
Apply Instruction
Send your CV to recruit@silvapharma.com
Company Information
Silva Pharmaceuticals Limited
How to apply on Silva Pharmaceuticals Limited
- At the very First, all you have to go to the Jiban Bima official website.
- JBC Job Apply Online
- Then you have to fill up the online form there
- You have to write down your father/mother/husband name, full address,
- Email ID, mobile number, district, Thana, union, word name,
- And respective community clinic name.
- After filling up the form, you have to submit it online.
- Then you will be provided the application ID and password.
- Finally, you have to download it and save it to get admit card.
Note: Private Company in Bangladesh. Bank Jobs Results. Government Jobs Results. Government jobs in Bangladesh. Bank jobs in Bangladesh. NGO jobs in Bangladesh. Private jobs in Bangladesh. Part-time jobs in Bangladesh. Marketing jobs in Bangladesh, IT Center jobs in Bangladesh. Pharma jobs in Bangladesh.
We trust that our distributing data helps the activity searchers who are finding a superior employment. We likewise share slanting assets for learner uniquely who is re-expanding their insight